Introduction
Background
Clinical laboratories (labs) analyze human specimens such as blood, tissue, and urine so that medical professionals can make diagnoses and prescribe treatment. As a part of the California Department of Public Health (Public Health) and under the direction of the Office of the State Public Health Laboratory Director, Laboratory Field Services (Laboratory Services) is responsible for licensing, registering, and overseeing labs. As of July 2015 Laboratory Services reported it was responsible for overseeing roughly 22,100 labs.
According to state law, the complexity of the tests that labs perform determines whether they must obtain licenses or registrations, as summarized in the text box. Of the approximately 22,100 clinical labs Laboratory Services was responsible for overseeing as of July 2015, about 2,800 were licensed and about 19,300 were registered. A license or registration is valid for one year, thus requiring annual renewal for the lab to continue operating.
Registration and Licensure Requirements for Clinical Laboratories
- Clinical laboratories (labs) requiring licensure perform tests of moderate to high complexity, such as testing for hepatitis or certain sexually transmitted diseases by DNA probe.
- Labs requiring registration perform simpler tests, with less chance of error or risk, such as prepackaged manufactured tests.
Sources: California Business and Professions Code, Section 1265, and Laboratory Field Services’ documents.
A lab seeking to obtain or renew a license or registration must pay a fee to Laboratory Services. Although registration fees are a set amount, each lab’s license fee is based on the volume of tests it conducts. Laboratory Services deposits the fees and other money it collects into the Clinical Laboratory Improvement Fund. The law states that the total fees Laboratory Services collects shall not exceed its costs for licensing, certifying, and inspecting labs, as well as performing other activities relating to the regulation of labs and lab personnel. For fiscal year 2013–14, Laboratory Services reported more than $6.5 million in fee revenue from licensed and registered labs.
At times, a medical professional located in California will send a specimen to a lab in another state or another country for analysis; these labs are referred to as out‑of‑state labs. State law requires that the receiving lab hold a license or registration from Laboratory Services. Further, out‑of‑state labs that Laboratory Services licenses rather than registers are subject to its periodic oversight, as described below.
State‑Mandated Responsibilities for Lab Oversight
The State has overseen labs since 1926 and has licensed labs since the 1950s. State law currently requires Laboratory Services to oversee labs by inspecting them, monitoring their proficiency testing, annually renewing their licenses and registrations, receiving and investigating complaints against them, and sanctioning those that violate laws or regulations. Laboratory Services must engage in two periodic oversight functions: conducting regular inspections and monitoring proficiency testing. According to state law, Laboratory Services must inspect each licensed lab every two years, notify the lab of any deficiencies the inspection reveals, and work with the lab to correct the deficiencies. Registered labs are not subject to routine inspections every two years under state law, but Laboratory Services may inspect them as part of complaint investigations.
Proficiency Testing Process for Licensed Clinical Laboratories
What Is Proficiency Testing?Proficiency testing is a process clinical laboratories (labs) use to verify the accuracy and reliability of their tests.
How Does Proficiency Testing Work?A provider distributes a specimen to a lab, which must evaluate the specimen and then submit the results to the provider. The provider has a target value for the specimen, and on receiving the lab’s assessment, the provider compares the lab’s results with its target value to determine if the lab’s evaluation was accurate.
How Often Must Labs Test?In general, labs must engage in proficiency testing at least three times a year.
What Is a Testing Failure?Participation is unsuccessful if the lab does not achieve a minimum score on either two consecutive tests or two out of three consecutive tests.
Sources: California Business and Professions Code, Section 1220, and Title 42, Code of Federal Regulations, Part 493.
The second type of periodic oversight Laboratory Services must perform is monitoring proficiency‑testing results. Proficiency testing provides an external evaluation of the accuracy of the labs’ test results. Licensed labs must participate because they perform complex tests; however, registered labs—which perform simple tests—are not required to participate in proficiency testing. The text box describes the proficiency testing process. Laboratory Services’ policy generally calls for it to receive and review each lab’s proficiency‑testing results at least three times a year and identify any instances of unsatisfactory performance. In those instances, according to its policy, Laboratory Services must notify the lab and require a plan of corrective action. If the planned corrective action is not acceptable or the lab’s test results do not improve, Laboratory Services can bar the lab from providing those test services.
Laboratory Services’ other oversight responsibilities include investigating complaints and issuing sanctions. State law requires Laboratory Services to investigate complaints it receives about labs and authorizes it to inspect labs as part of its complaint investigations. Further, when labs do not adhere to state law and regulations, Laboratory Services has the authority to issue sanctions that can include monetary penalties, plans of correction, and license or registration revocation. If Laboratory Services revokes a lab’s license or registration, the lab’s owner and operator are automatically barred from owning or operating a lab for two years.
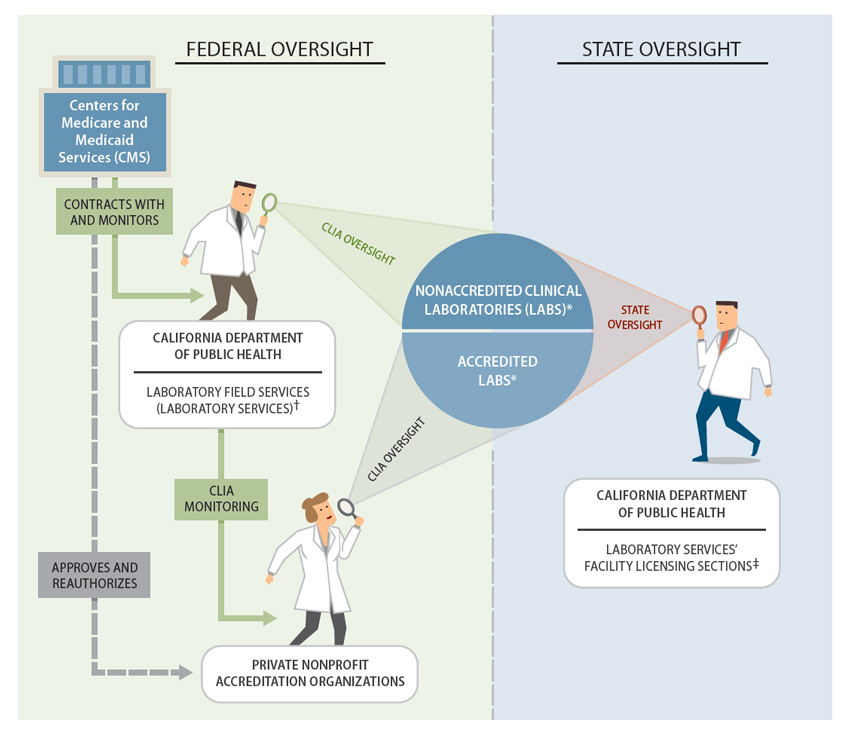
Laboratory Services has offices in Richmond and Los Angeles. It divides the licensing, registration, and oversight functions it is mandated to perform between the two locations. Figure 1 is a partial depiction of Laboratory Services’ organizational structure. As the figure shows, two of Laboratory Services’ sections perform functions related to the state mandates for labs. One section, located in Los Angeles, oversees federal lab requirements, as described below.
Figure 1
Partial Depiction of Laboratory Field Services’ Organizational Structure as of February 2015
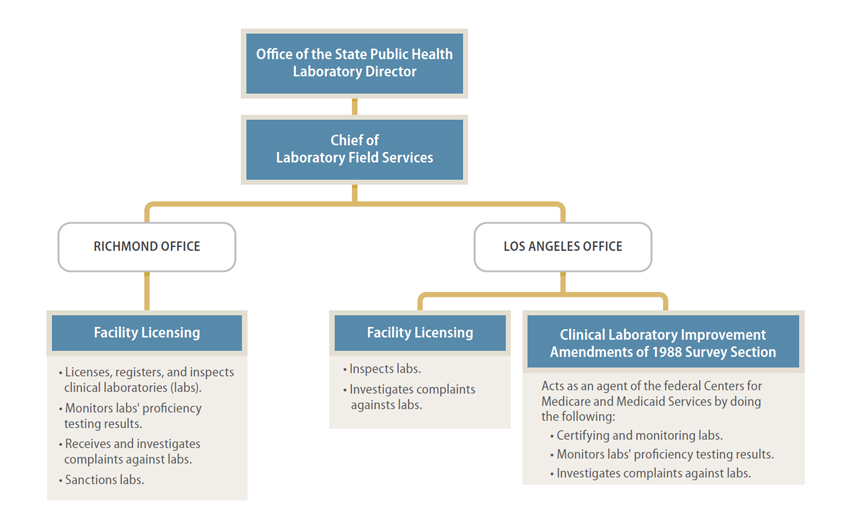
Sources: Laboratory Field Services’ organization chart dated February 18, 2015, and the California State Auditor’s analysis of functions assigned to each section.
Federal Oversight of Labs
In addition to meeting state requirements, all the labs that Laboratory Services licenses or registers must also follow federal regulations. The Clinical Laboratory Improvement Amendments of 1988 (CLIA) is a federal law enacted to ensure the accuracy and reliability of lab testing. This law extended federal regulation for the first time to all labs in the nation that perform tests on human specimens for medical diagnosis, treatment, or health assessment. The federal Centers for Medicare and Medicaid Services (CMS) has primary responsibility under CLIA for regulating approximately 250,000 labs nationwide as of November 2014. CMS meets this responsibility in part by contracting with state agencies across the country to monitor and enforce compliance with CLIA. By law, activities to enforce CLIA requirements must be self‑funded. With few exceptions, labs must apply for a CLIA certificate and pay a biennial fee to cover the cost of inspections and other regulatory activities.
CLIA groups labs into two categories—those performing simple tests, such as urine dipstick tests and finger‑stick blood tests, and those performing moderately complex to highly complex tests (complex tests). A lab’s category dictates the federal oversight to which it is subject. CLIA exempts labs from virtually all federal rules if they perform only simple tests in strict compliance with the manufacturers’ instructions. However, as Figure 2 shows, labs that perform complex tests differ from those performing simple tests in two ways: They are subject to ongoing oversight in the form of biennial inspections and proficiency testing, and they can choose their oversight body.
Figure 2
Clinical Laboratory Improvement Amendments of 1988—Requirements and Oversight
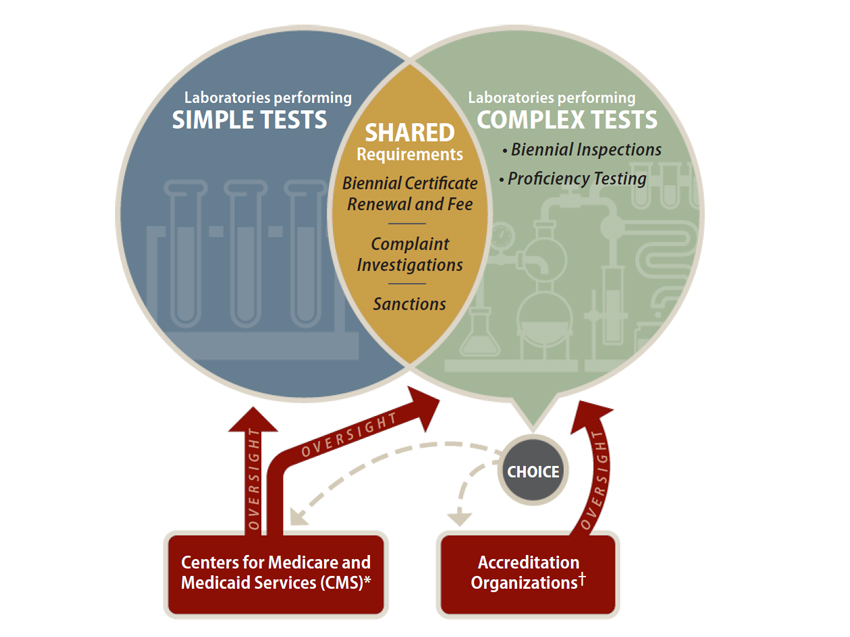
Source: Title 42, Code of Federal Regulations, Part 493.
* CMS has primary responsibility for administering the Clinical Laboratory Improvement Amendments of 1988, which it accomplishes through
contracts with state agents.
† Accreditation organizations chosen to oversee a licensed clinical laboratory must be approved by CMS.